

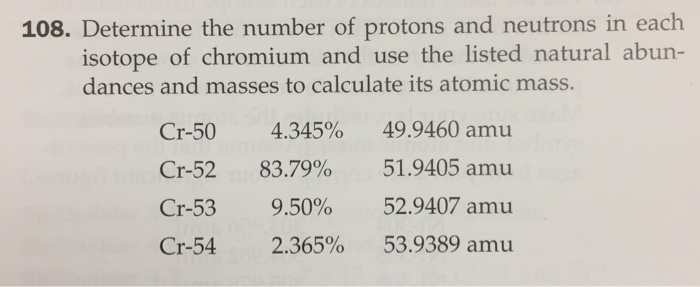
A radioactive isotope is one that breaks apart and gives off some form of radiation. … Seven radioactive isotopes of chromium are known also. There are four naturally occurring isotopes of chromium: chromium-50, chromium-52, chromium-53, and chromium-54. Read More: What is a belaying pin 5e? Is chromium a radioactive element? Cr-53 and Cr-54 are used for the study of chromium metabolism and studies into (adult) diabetes.

Cr-50 is used for the production of the radioisotope Cr-51 which is used for measuring blood volume and red blood cell survival. Several Chromium isotopes are used for medical applications. N.Vauquelin in the mineral crocoite, which is a natural form of lead chromate, PbCrO 4. Of the six artificial radioactive chromium isotopes, the most important is 51Cr, with a half-life of 27.8 days, which is used as an isotopic tracer. Use GM detector or liquid scintillation counter to detect Cr-51.Wholebody and ring badges are required for work with Cr-51.Follow General Precautions for working with radioactive material.How can you protect yourself from chromium 51? … iron 59 a radioisotope of iron having a half-life of 44.5 days used in ferrokinetics tests to determine the rate at which iron is cleared from the plasma and incorporated in red blood cells. Is Chromium 51 natural or synthetic?Ī synthetic radioactive isotope of chromium having a half-life of 27.7 days and decaying by electron capture with emission of rays (0.32 MeV) it is used to label red blood cells for measurement of mass or volume, survival time, and sequestration studies, for the diagnosis of gastrointestinal bleeding, and to label … How is iron 59 used for diagnosis?įrom the intestine, iron is transported on transferrin to the liver or the bone marrow. They give off their radiation at a low dose rate over several weeks, and then the seeds can remain in the prostate gland permanently. Gold-198 seeds are used in permanent seed implant therapy involving injecting approximately 30-100 radioactive seeds into the prostate gland. Read More: Does the Boston Latin School still exist? What is the structure of chromium 51?Ĭhromium-51 is a synthetic radioactive isotope of chromium having a half-life of 27.7 days and decaying by electron capture with emission of gamma rays (0.32 MeV) it is used to label red blood cells for measurement of mass or volume, survival time, and sequestration studies, for the diagnosis of gastrointestinal … What is the use of gold 198? What does Chromium 51 give off?Ĭhromium-51 is a commonly used radionuclide with a half-life of 27.7 days, emitting gamma rays with a maximum energy of 0.320 MeV (Million Electron Volts). Chromium 51 is also used for labeling platelets to study their services. Where is chromium 51 used in medicine?Ĭhromium 51 is used for the labeling of red blood cells for the evaluation of mass or volume, survival time and sequestration studies, and for the diagnosis of gastrointestinal bleeding. Cr is produced in a reactor by neutron activation.

Advantages of this radionuclide include ease of red cell labeling, excellent red cell uptake, low toxicity, and low and stable elution rate.
What are the benefits of using chromium 51?Ĭr is a useful red cell label and also has utility as a platelet label.


 0 kommentar(er)
0 kommentar(er)
